Safety Profile
Safety Profile
The following data reflect exposure to WINREVAIR in the pivotal STELLAR trial.
- After completing the primary 24-week treatment phase, patients continued into a long-term double-blind (LTDB) treatment period, maintaining their current therapy, until all patients completed the primary treatment period.
- The median durations of treatment were similar between the placebo and WINREVAIR groups (229.5 days vs 252.0 days, respectively).
Adverse reactions occurring in STELLAR by the time all patients completed the primary 24-week period of the study are summarized below.
Adverse reactions in patients receiving WINREVAIR (DBPC + LTDB)a:
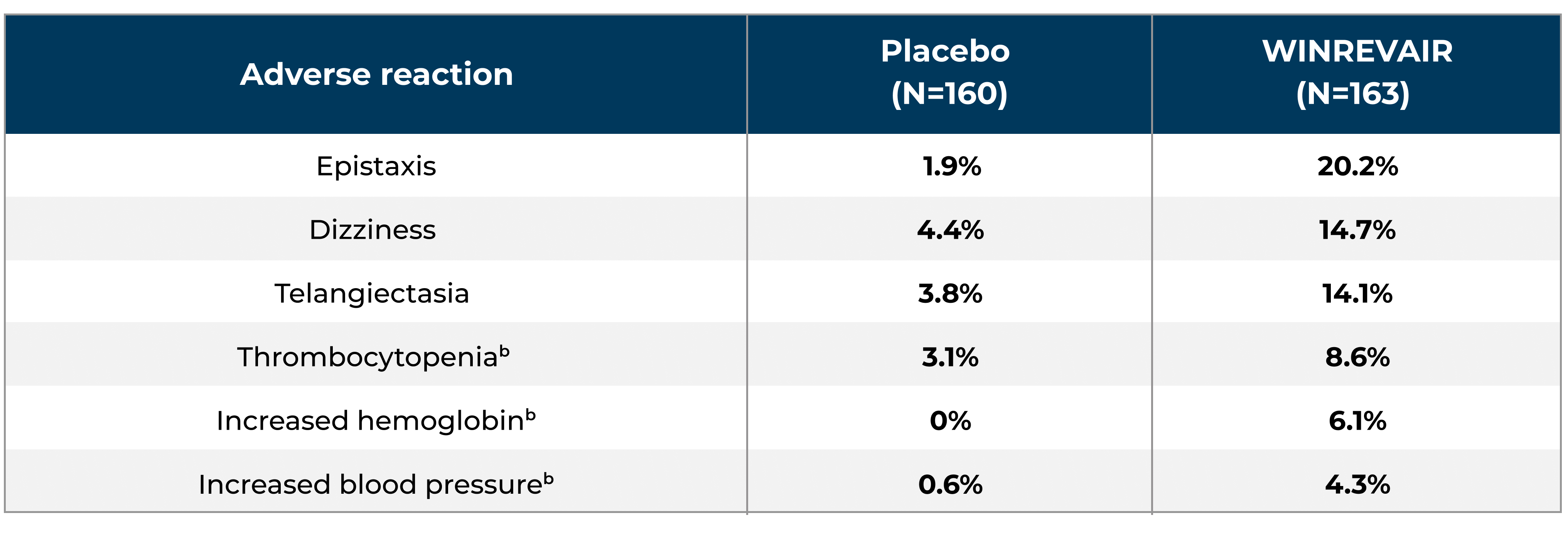
aDouble-blind placebo-controlled period + long-term double-blind period.
bComposite terms.
Increased hemoglobin
The majority of events of increased hemoglobin (Hgb increased, polycythemia) were non-serious, mild, and reversible, and were not associated with discontinuation of therapy.
- Moderate elevations in Hgb (>2 g/dL above upper limit of normal [ULN]) occurred in 12.3% of patients taking WINREVAIR.
- No severe elevations (≥4 g/dL above ULN) were observed.
- Increases in Hgb were manageable by dose delays, dose reductions, or both.
Thrombocytopenia
The majority of events of thrombocytopenia (thrombocytopenia and platelet count decreased) were non-serious, mild, reversible, and have not been associated with discontinuation of therapy.
- Severe reduction in platelet count <50,000/mm3 (<50.0 x 109/L) occurred in 1.8% of patients taking WINREVAIR.
- Thrombocytopenia occurred more frequently in patients also receiving prostacyclin infusion (10.8% taking WINREVAIR and 0% taking placebo) compared with patients who were not taking prostacyclin infusion (3.1% of patients taking WINREVAIR and 3.1% of patients taking placebo).
Telangiectasia
Events of telangiectasia were non-serious and did not progress in severity over time.
- In all patients exposed to WINREVAIR, the median time to onset was 47.1 weeks.
- Discontinuations of therapy due to telangiectasia were 1% in the WINREVAIR group vs 0% in the placebo group.
- No episodes of serious bleeding have been associated with telangiectasia.
Increased blood pressure
Events of increased blood pressure (hypertension, blood pressure diastolic increased, blood pressure increased) were non-serious and no severe events were reported.
- In patients taking WINREVAIR, mean systolic blood pressure increased from baseline by 2.2 mmHg and diastolic blood pressure increased by 4.9 mmHg at 24 weeks.
- In patients taking placebo, the change from baseline in mean systolic blood pressure was -1.6 mmHg and -0.6 mmHg change in diastolic blood pressure.
Treatment discontinuation
The overall incidence of treatment discontinuations due to an adverse reaction was 4% in the WINREVAIR group and 7% in the placebo group.
There were no specific adverse reactions causing treatment discontinuations that occurred with a frequency greater than 1% and more often in the WINREVAIR group.
Long-term safety data
Long-term safety data are available from a Phase 2 clinical trial (PULSAR) that comprised a 24-week, double-blind, placebo-controlled treatment period followed by a 30-month, open-label extension period (n=104). A majority of these patients then continued into a long-term follow-up study.
- The mean duration of exposure to WINREVAIR in PULSAR and the long-term follow-up study was 151 weeks, with a maximum exposure of 218 weeks.
- The safety profile was generally similar to that observed in the pivotal STELLAR study.
- However, telangiectasia was not observed during the double-blind, placebo-controlled treatment period in PULSAR.
- Telangiectasia was first reported in the open-label extension, occurring in 27% of patients at study completion, with a median time to onset of 106 weeks.
[SSI PLACEHOLDER]
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Vestibulum in nu Ila odio. Donec vitae libero hendrerit, tincidunt erat a, aliquam arcu. Proin vel dictum risus. Nunc aliquam pellentesque magna, ut dignissim mi viverra sed. Nunc eget dolor quis erat rhoncus varius ut vel urna. Etiam commodo odio felis, nee mattis ex lobortis quis. Nulla eleifend eleifendjusto. Phasellus varius tempor lacinia. Suspendisse lacus ante, consequat nee orci et, congue dictum lectus. Phasellus lectus nisi, maximus ac sem ut, varius blandit sapien. Nam efficitur sapien sed dui mattis, nee varius elit egestas. Mauris commodo nee neque sit amet sodales.
Ut congue imperdiet diam, non faucibus sapien finibus non. Maecenas lacus mi, sodales non convallis non, malesuada at mi. Maecenas risus eras, luctus eget dapibus quis, semper vel massa. Phasellus euismod tortor id tortor interdum maxim us. Etiam sodales erat sit amet quam sollicitudin hendrerit. Sed sollicitudin lectus ut mi lobortis, vel efficitur arcu ullamcorper. Nam aliquam eget ligula sit amet feugiat. Sed vulputate, arcu et cursus ultrices, velit nisl feugiat mauris, ut eleifend ante dolor vitae massa. Praesent lacinia purus leo, quis porta ipsum commodo in. Donec pretium leo ante, id faucibus purus bibendum id. Donec iaculis augue augue, aliquet pulvinar mi vehicula in. Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas. Phasellus enim massa, tempor non elit congue, rutrum rhoncus nulla. Donec finibus eu ipsum vitae porttitor. Vestibulum tincidunt tincidunt quam, id lobortis eras luctus ac. Pellentesque faucibus lacus vel elit viverra fringilla.
